INL Researchers publish a paper about the relevance of CTC classification to subtype metastatic breast cancer patients and update their treatment
Breast cancer is the most prevalent type of cancer worldwide. By late 2020, there were 7.8 million women alive who had been diagnosed with breast cancer during the previous 5 years. Clinical management and technological advancements allow most primary and early-stage breast cancers to be treated, either by surgery alone or surgery and complementary therapy, achieving an overall 5-year survival rate of 90 %. Nevertheless, when cancer spreads and metastasis occurs, the 5-year survival rate drops to 26 %. Existing targeted therapies have significantly improved patient outcomes; still, designing these personalized treatments relies on accurate and comprehensive assessment of cancer alterations during time, that cannot only depend on invasive tumour biopsies.
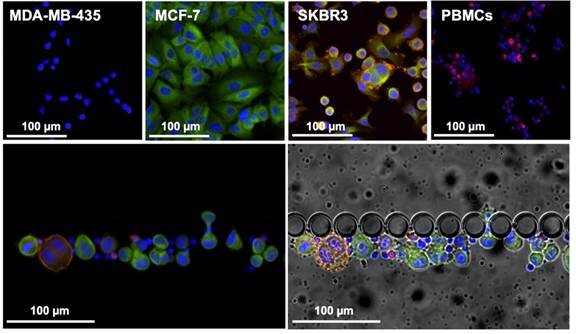
Liquid biopsy is a great alternative, since it is a minimally invasive and painless method to provide continuous, reliable and real-time information on the tumour progression through the molecular analysis of circulating biomarkers. Circulating tumour cells (CTCs) present the same phenotype and genotype as the active tumour from where they originate and, as such, their continuous analysis can provide information about the disease in real-time, allowing a more accurate prognosis and being an ideal approach for early detection of metastasis.
In this article, authors from INL, iMM, together with the oncology department of the Hospital de Santa Maria in Lisbon, and INL’s spin-off company RUBYnanomed, used a novel microfluidic device, the RUBYchip™, to isolate and enumerate CTCs from metastatic breast cancer patients. They assessed their HER2 expression in a comparative study with the current gold standard technology and demonstrated relevance of monitoring Circulating Tumor Cells (CTCs) and their subtype to predict treatment resistance in metastatic breast cancer. The technology developed at INL for CTC isolation and now transferred to RUBYnanomed demonstrated superior efficiency and sensitivity for the isolation and classification of CTCs compared to existing clinical tools. We expect this technology will help the sub-classification of metastatic breast cancer patients and update their treatment.
The authors would like to send a heartfelt thank you to the patients and healthy volunteers who kindly and generously participated in this study.
Read the publication here.